A recent Twitter thread:
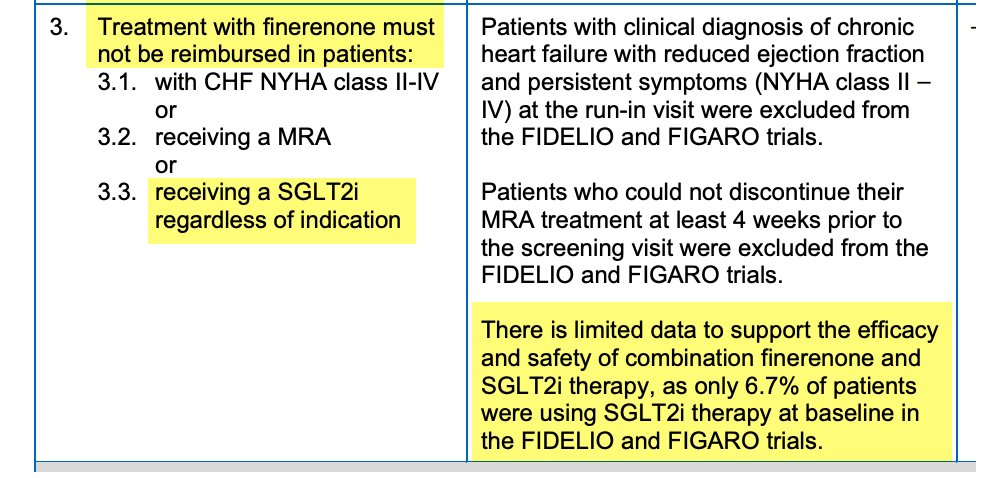
As a Canadian nephrologist, relevant & active national debate in light of CADTH draft guidelines which suggest finerenone not be funded if on an SGLT2i.
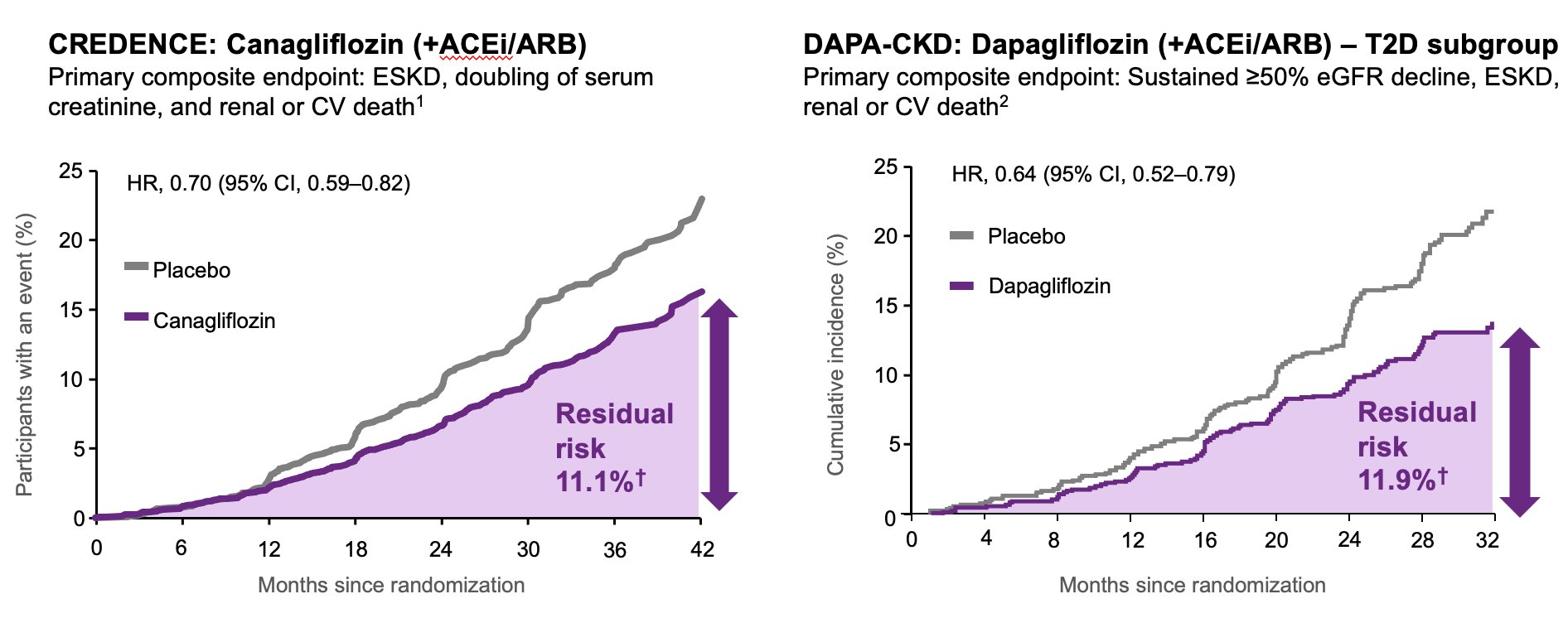
We know from CREDENCE, DAPA-CKD and EMPA-KIDNEY that SGLT2i + ACEi or ARB (aka RAASi) is safe and effective at reducing renal/CV endpoints.
Finerenone was studied in FIDELIO and FIGARO & enrolled when SGLT2i were not yet standard care.
These trials showed that finerenone + RAAS blockade reduced cardiovascular and renal endpoints There are no trials yet that evaluate triple therapy (Finerenone + SGLT2i + RAASi) vs dual therapy (SGLT2i + RAASi).
Even in patients in the active treatment arm in CREDENCE, DAPA-CKD and EMPA-KIDNEY, there was significant residual risk of adverse events While treatment with dual therapy is good, it is not sufficient
2022 KDIGO guidelines indicate that finerenone can be added to a RAS inhibitor and SGLT2 inhibitor for treatment of CKD and T2D.
“the most logical application of finerenone is to patients with high residual risks of CKD progression and [CV} events, as evidenced by the presence of albuminuria (ACR >30 mg/g [>3 mg/mmol]) despite lifestyle modifications and first-line drug therapies” – KDIGO 2022 CPG
KDIGO takes into account the 877 FIDELIO/FIGARO participants on an SGLT2i at baseline and the complementary mechanisms of action which suggest that benefits of SGLT2i and finerenone may be additive
KDIGO specifically notes the pre-specified individual patient-level combined analysis of the FIDELIO and FIGARO trials shows no significant heterogeneity in the cardiovascular or renal benefit in those using an SGLT2i at baseline
KDIGO notes the possibility that concurrent use of finerenone with SGLT2 inhibitor may reduce hyperkalemia.
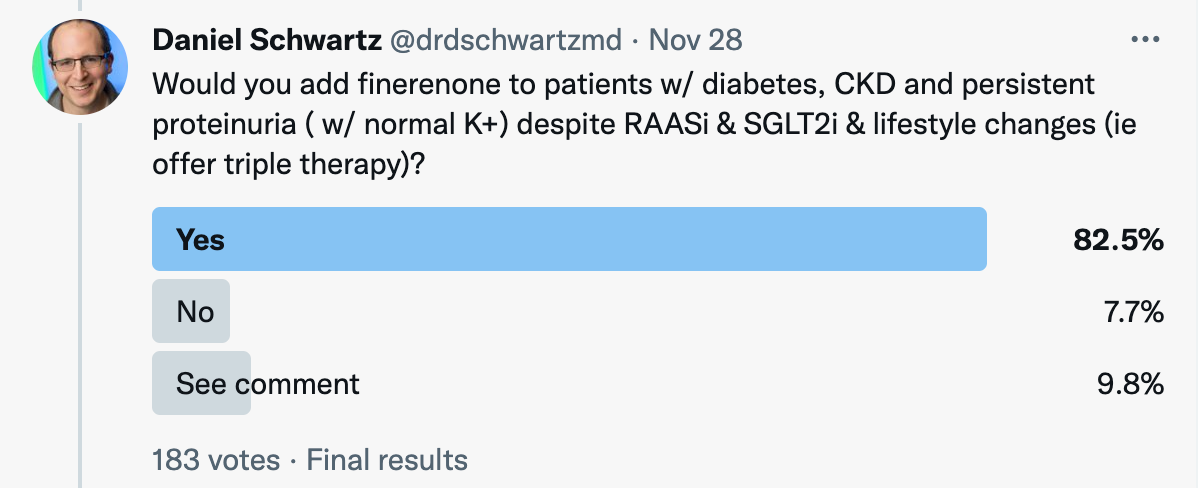
When the Twitter community was asked: “Would you add finerenone to patients w/ diabetes, CKD and persistent proteinuria ( w/ normal K+) despite RAASi & SGLT2i & lifestyle changes (ie offer triple therapy)?”, survey results were as follows:


Recent Comments